Novavax Covid Vaccine Side Effects
NVAX a biotechnology company developing next-generation vaccines for serious infectious diseases today announced the publication of results from the final analysis of a pivotal Phase 3 clinical trial of its COVID-19 vaccine candidate conducted in the United Kingdom in the New England Journal of Medicine NEJM. Lower risk of side effects may encourage vaccination among lower-income individuals who cant afford to miss a days worth of pay from work.
Novavax Large Study Finds Covid 19 Shot About 90 Effective
We know vaccines are working against new COVID.

Novavax covid vaccine side effects. 28 that its vaccine was more than 89 effective in protecting against Covid-19 in its phase three clinical trial conducted in. Novavax has for years worked on developing its recombinant nanoparticle technology and created the first COVID-19 vaccine using this method in the spring of 2020. Its side effects are relatively mild and like those of the other vaccines such as pain and tenderness at the injection site fatigue headache and muscle pain.
Indias drug regulator has given the green signal to the vaccine manufacturer according to a report by Times Of India. Side effects are a big barrier for COVID-vaccine acceptance. Novavax a small American company buoyed by lavish support from the US.
Heres why scientists are stoked about it. It may do so by July at the earliest and possibly as late as late September. Manufacturing regulatory issues have prevented Novavax from seeking emergency use authorization from the FDA.
Novavaxs investigational vaccine NVX-CoV2373 is made from a stabilized form of the coronavirus spike protein using the companys recombinant protein nanoparticle technology. Government announced on Monday the results of a clinical trial of its Covid-19 vaccine. Everything You Need to Know About Novavax the New COVID Vaccine Novavax a fourth COVID-19 vaccine could soon be approved in the United States.
Side effect of Pfizer still being studied Vaccine experts in the Philippines meanwhile are still evaluating the reported heart inflammation among recipients of Pfizer COVID-19 jabs. The CDC reported on. The decision holds huge significance considering the probable third wave of Covid which is likely to affect children as claimed by top.
Injection site pain and tenderness as well as fatigue headache and muscle pain were the most commonly reported side effects. Based on Novavaxs phase 3 clinical trials their COVID-19 vaccine appears to have a substantially lower rate of side effects than the Pfizer-BioNTech or Moderna vaccines. Preliminary phase 3 results indicate that side effects following administration of the Novavax COVID-19 vaccine have been generally mild to moderate and short-lived.
PackageLabel Display Panel Vial Label. The Serum Institute of India has got the nod from DCGI to conduct trials of the Novavax Covid-19 vaccine on children aged 7-11. The Novavax vaccine appeared to trigger fewer mild-to-moderate side effects in its trials than.
What about side-effects. But in an agreement with Takeda the company hopes to. If the FDA sees no urgency the Novavax vaccine might not be available in the US.
The purified protein antigens in the vaccine cannot replicate and cannot cause COVID-19. The vaccine also contains a proprietary adjuvant MatrixM. By summer 2020 early clinical trials showed that the vaccine appeared to be safe and more advanced trials entered the planning stage in the United States and other countries.
In a Reuters report Novavax said its protein-based vaccine was more than 93 effective against the more contagious predominant coronavirus variants. GAITHERSBURG Md June 30 2021 PRNewswire -- Novavax Inc. COVID-19 vaccine side effects are either a physical manifestation of your bodys immune response which is the case for most people or an allergic reaction said Jesse Erasmus acting assistant professor in the department of microbiology at the University of.
Suspension for intramuscular injection Multi-dose vial 10 doses of 05 mL Record datetime of first use. After the latters attempt at developing a COVID-19 vaccine. Novavaxs side effects are relatively mild and similar to commonly reported side effects for Pfizer Moderna and Johnson Johnson.
Novavax is behind schedule in applying for regulatory approval for its COVID-19 vaccine.
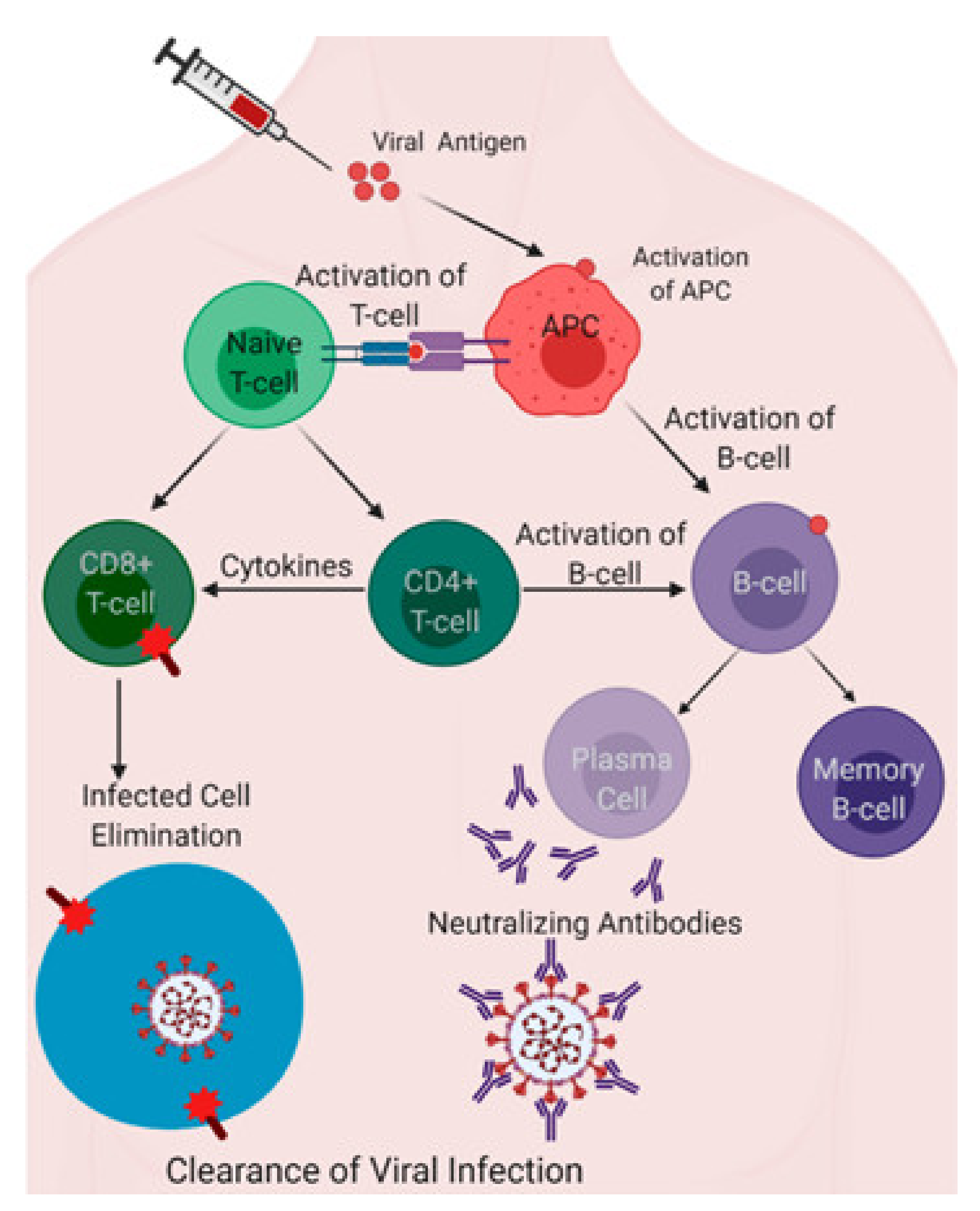
Pathogens Free Full Text A Summary Of The Sars Cov 2 Vaccines And Technologies Available Or Under Development Html

Farmacovigilancia De Vacunas Para Covid 19 Novavax

Novavax Stock Falls 23 On Covid 19 Treatment Optimism Time To Buy Trefis
Study Finds Novavax Covid 19 Vaccine About 90 Effective King5 Com

Pathogens Free Full Text A Summary Of The Sars Cov 2 Vaccines And Technologies Available Or Under Development Html

Study Finds Novavax Covid 19 Vaccine About 90 Effective King5 Com

How The Moderna Astrazeneca Pfizer And Other Covid Vaccines Compare
Novavax Expects Covid 19 Vaccine Trial Results In July

Novavax Seeks Covid 19 Vaccine Use In India Indonesia The Philippines Ahead Of U S Cp24 Com
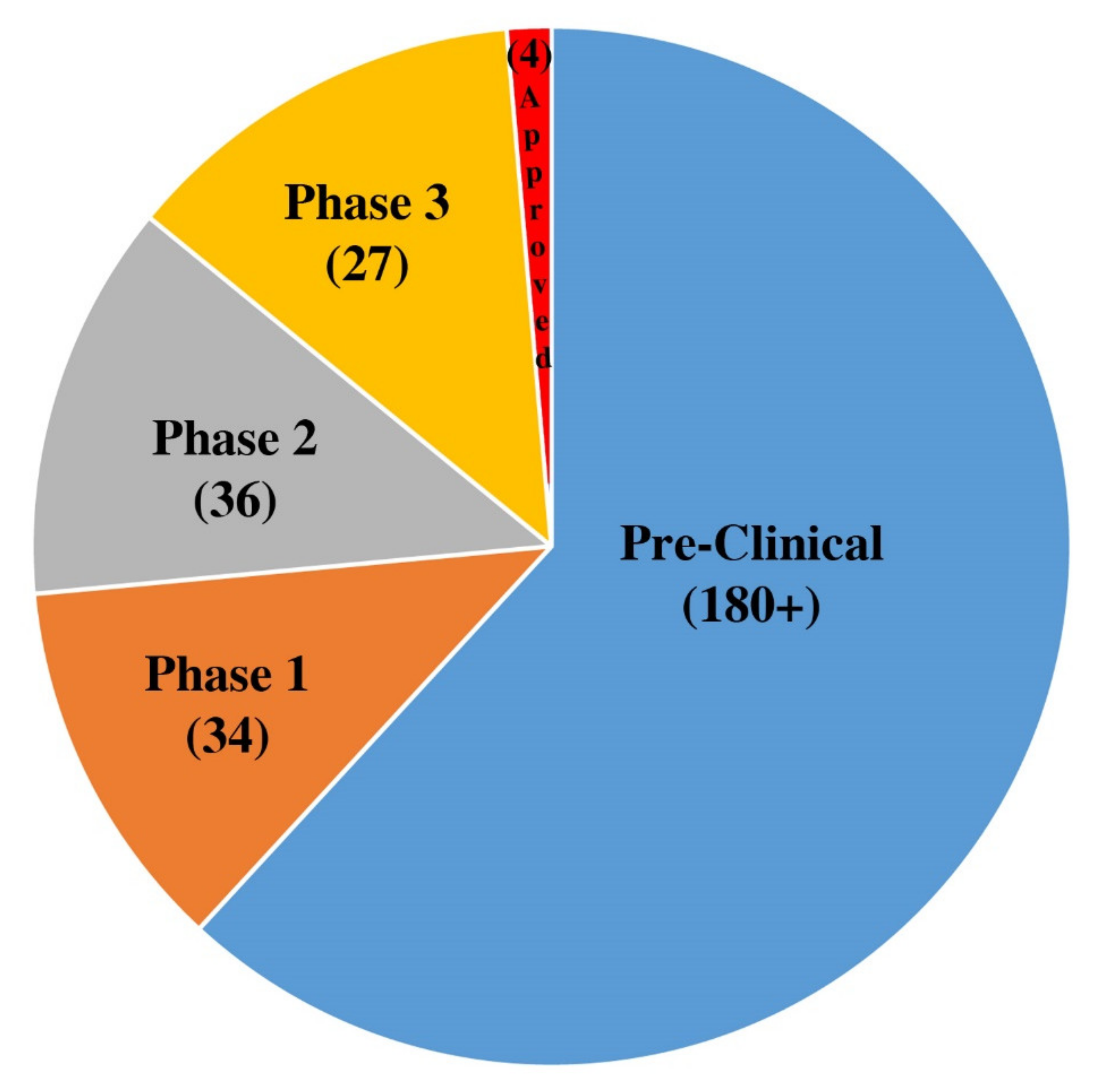
Pathogens Free Full Text A Summary Of The Sars Cov 2 Vaccines And Technologies Available Or Under Development Html
Racgp Atagi Recommends Major Change To Vaccine Rollout
Which Covid Vaccine Is Best Does It Make A Difference The Independent

Farmacovigilancia De Vacunas Para Covid 19 Novavax

Farmacovigilancia De Vacunas Para Covid 19 Novavax
Novavax Expects Covid 19 Vaccine Trial Results In July
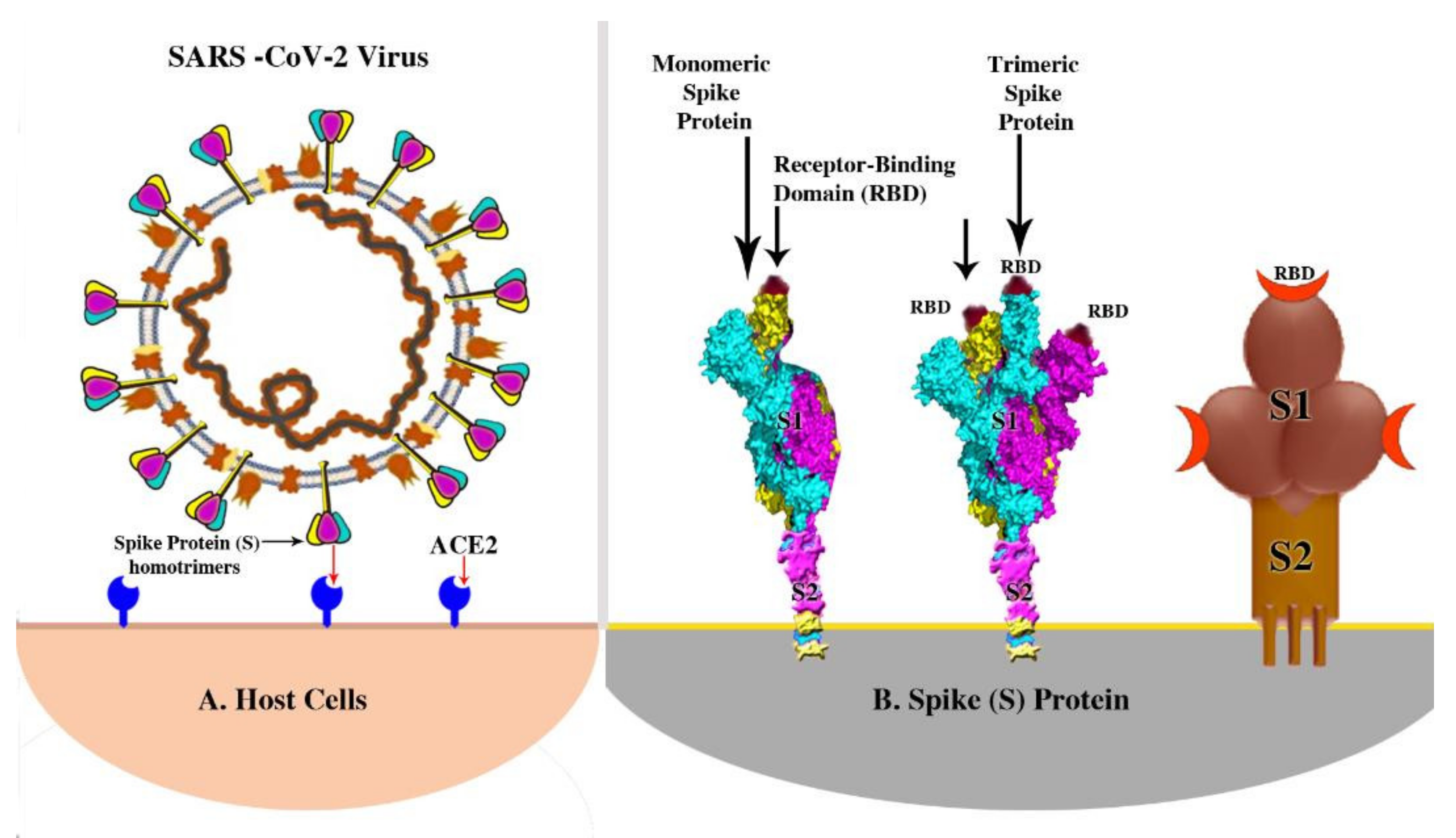
Pathogens Free Full Text A Summary Of The Sars Cov 2 Vaccines And Technologies Available Or Under Development Html



